Program

The Frontiers in Chemistry Master’s Program results from the creation of Université Paris Cité. The molecular Chemistry towards Life’ sciences developed on the campus Saint-Germain-de-Prés answers to the physical chemistry oriented towards nanoscience, surfaces and energy developed on the Campus des Grands Moulins…. The Master’s program is supported by high-level research laboratories with international reputation and network.
The main objective of the first year is to provide students with a strong background in the various aspects of molecular chemistry, physical chemistry and reactivity at the interfaces with materials, surface and life sciences. Theoretical knowledge acquired through tutoring and analysis of scientific articles is complemented by a 2 to 5 months internship. Through the choice of optional courses, students can prepare their second-year specialization. The second year of the Master is intended to specialize students’ capacities in electrochemistry, spectroscopies, environmental chemistry or molecular chemistry. Students must choose among four tracks, two optional teaching units and will perform a 6 months internship to complete the Master’s Degree.
| M1 | S1 | Common core (15 ECTS) | |||
| Molecular Chemistry (15 ECTS) | Physical Chemistry (15 ECTS) | ||||
| S2 | Common core (9 ECTS) | ||||
| OBMC (12 ECTS) | PC Life (12 ECTS) | CHENS (12 ECTS) | (Closed from 2024) | ||
| Internship (2-5 months, 9 ECTS) | |||||
| M2 | S3 | Common core (15 ECTS) | |||
| OBMC (15 ECTS) | PC Life (15 ECTS) | CHENS(15 ECTS) | (Closed from 2024) | ||
| S4 | Common core (15 ECTS) | ||||
| Internship (5 months, 26 ECTS) | |||||
SEMESTER 1
COMMON CORE
OPTION 1 – Molecular Chemistry
OPTION 2 – Physical Chemistry
SEMESTER 2
COMMON CORE
– Vibrational and optical spectroscopies
– 2 to 5 months internship in research laboratories or industries, in France or abroad
OPTION 1 – Organic, Bioorganic Medicinal Chemistry / OBMC
– Medicinal & heterocyclic chemistry
– Advanced technical lab skills
OPTION 2 – Physical Chemistry for Life Sciences / PC-Life
– Advanced NMR and mass spectroscopy techniques – UE mutualized with Anal-Chem track
– Biotechnologies (practicals)
– Basis of bio-mollecular recognition
– Synthesis of nano-objects – UE mutualized with CHENS track
OPTION 3 – Analytical Sciences for Environment / SAFE (Closed from 2024)
– Elemental analysis & environnement 1
– Elemental analysis & environnement 2
– Environment, Structure and Processes
– Advanced NMR and mass spectrometry – UE mutualized with Chem-Life track
OPTION 4 – Analytical Sciences for Environment / CHENS
– Materials & Functional Nanomaterials
– Electrochemistry for Energy Conversion and Storage
– Numerical chemistry
– Synthesis of nano-objects – UE mutualized with Chem-Life track
SEMESTER 3
COMMON CORE
– Research seminars
– Computational and modeling tutored project
– Collaborative research project – Part 1
– Option (2 courses to be picked among the list « optional teaching units » below)
ITINERARY OBMC – ORGANIC BIOORGANIC MEDICINAL CHEMISTRY / OBMC
– Selective methods for medicinal chemistry
– Click chemistry and bioconjugaison for biosensor
– Chemistry of RNA, DNA and proteins for new therapeutic strategies
– Bio-inorganic chemistry: tutored project
– Investigating molecular structure, dynamics and interactions by NMR
ITINERARY CHENS – CHEMISTRY FOR NANOSCIENCES AND ENERGY / CHENS
– Near Field Microscopies in Nanochemistry
– Surfaces and Nanomaterials Characterization – UE mutualized with SAFE track
– Molecular and Organic Electronics
– Transduction of solar energy: from (bio) molecules to nanomaterials
ITINERARY PHYSCHEMLIFE – PHYSICAL CHEMISTRY FOR LIFE SCIENCES / PC-Life
– Optical Spectroscopic methods for Biomolecule Analysis
– Advanced Biophysical Techniques
– Nanomedicine: from guiding principes to engineering of nanomaterials for therapy
– Bioimaging: Advanced techniques and development of (nano-)tracers
– Biomolecular Diagnostic: from basic concepts to biosensing
ITINERARY SAFE – ANALYTICAL SCIENCES FOR ENVIRONMENT / SAFE (Closed from 2024)
– Analyse of elements and isotopes
– Quantitative organic analyses
– Chemiometrics in heterogeneous and complex systems
– Environmental pollution, Health and the Biosphere
– Surfaces and Nanomaterials Characterization – UE mutualized with CHENS track
OPTIONAL TEACHING UNITS
– New tools and technologies in analytical chemistry, advanced determinations
– Functional Nanoparticles and Nanostructures
– Advanced computational project / Molecular modeling
– Green chemistry : recent updates
– Synthesis of bioactive compounds
– Medicinal chemistry
SEMESTER 4
COMMON CORE
![]()
Course Title: Using Laser and Light to do Chemistry
Course supervisor:
- N. Felidj
- M. Robert
Goals: This course is intended to give an introduction to modern experimental spectroscopic techniques for the study of molecular and supramolecular systems, as well as the chemical reactivity of these systems, using light as induction method. For this, we will take advantage of examples of state-of-the-art research in experimental spectroscopy (all wavelength domains). Emphasis will be laid on light sources used in modern spectroscopy, such as lasers. Special attention will also be paid to scientific methodologies that couple spectroscopy to theoretical chemistry. Applications to chemical reactivity and catalysis in organic chemistry will illustrate these topics.
Description:
- Lasers as tools in physical chemistry and spectroscopy
(1 ECTS, 6h) – N. Félidj
- General principles, gas laser, solid state laser, diode laser, quantum cascade laser.
- Time resolved spectroscopy.
- Surface-enhanced spectroscopies (Raman,…).
- Modelling the photochemical reactivity with calculations
(1 ECTS, 6h) – A. Perrier
- Ab-initio methods for excited states
- Potential energy surfaces, ground and first excited states, photochemical reaction path
- Dynamical aspects
- An application, the photoreactivity of azobenzene
- Applications to chemical reactivity and catalysis
(1 ECTS, 6h) – J. Bonin, M. Robert- Investigating chemical reactivity with laser light, the case of proton-coupled electron transfer process in chemistry and biology
- Photoredox catalysis using visible light, activation of small molecules and organic substrates
Acquired skills: Knowledge of the most recent techniques of spectroscopy in research laboratory. Applications to the study of chemical reactions and catalytic process in organic chemistry.
Total number of hours: 18 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: Knowledge in spectroscopy M1 level.
Assessment: Final exam (100%)
![]()
Course Title: New tools and technologies in analytical chemistry, advanced determinations
Course supervisor:
Title: Dr.
First Name: Aline
LAST NAME: Gratien
Description: The objectives of this course is to know the capability and limitations of analytical instruments, choose the right method for the appropriate problem. This course will present techniques, methods and principles at the more advanced state of the art level for operating chemical analyses for the different phase (gas, water, soil, rock, materials, particle..). The teaching team (A. Gratien, Y. Sivry, R. Losno, M. Benedetti, P. Cartigny…) is wide for this title because the covered domain is large and needs specific knowledge.
Keywords: ultra-trace analyses, isotope caracterisation, inorganic and organic measurement
Total number of hours: 16 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: The content of the courses will be adapted to the knowledge and background of the students. The basics will be introduced at the beginning of each taught part. Nevertheless basic knowledge (theoretical background) on chemistry and analytical chemistry (M1 level) are required to understand the course content and to attempt to this UE.
Teaching methods and activities: Lectures (CM)
Assessment: final exam (100%)
![]()
Course Title: CHEMISTRY OF BIOMOLECULES
Course supervisor:
Title: Pr.
First Name: Mélanie
LAST NAME: Etheve-Quelquejeu
With the full participation of Dr. Patricia Busca
Description: Biomolecules including proteins, carbohydrates, lipids, and nucleic acids are an important element of living organisms. Currently, these molecules offer tremendous potential for biotechnology, new therapeutic strategies, or synthetic biology. Many pharmaceutical companies and academic groups are entering or investing in this field, and the chemists have already played a major role in the most significant developments.
The aim of this course is to address the specific problem of these biomolecules synthesis (peptides, sugars, and nucleic acids) with usual notions of organic chemistry.
We hope that this course will inspire future chemists to branch out on this topical field of designing new biomolecules for potent applications.
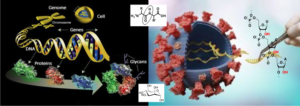
Contents:
Sugars: monosaccharide chemistry (protective groups), formation of the O-glycosidic bonds (methods of activation, control of stereochemistry, mechanistic aspects).
Peptides: Formation of peptide bonds, strategy of synthesis, synthesis on solid support.
Nucleic acids: Protective groups, Phosphodiester bond formation, solid support synthesis.
Keywords: peptides, sugars and nucleic acids chemistry
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Exact location: Campus Saint Germain des Prés
Prerequisites/skills needed: Basics in organic chemistry and molecular biochemistry
Teaching methods and activities: Lectures (CM), practical sessions (TD), other : reverse pedagogy
![]()
Course Title: New tools and technologies in analytical chemistry, advanced determinations
Course supervisor:
Title: Dr.
First Name: Aline
LAST NAME: Gratien
Goals: Knowledge of the capability and limitations of analytical instruments. Choosing the right method for the appropriate problem. The student will work on a proposed personal research project.
Description: This course will present techniques, methods and principles at the more advanced state of the art level for operating chemical analyses. The teaching team is wide for this title because the covered domain is large and needs specific knowledge.
Part 1: Instrumental techniques
- Separation of analytes (DJ, 2h CM)
- Inorganic and organic carbon measurements, isotopic determinations (MB, 2h CM)
Part 2: Analytical issues
- Ultra-trace analyses (RL, 2h CM)
- Measuring isotopes. (YS, 2h CM)
- Coupling separation and analyses (MB/DJ, 2h CM)
Part 3: New trends in instrumentation
- Single-particle ICPMS for nanoparticles detection (YS, 2h CM)
- Future needs for science and society (AG, 2h CM)
Acquired skills: Knowledge of the most recent techniques for analytical material and methods with an emphasis to inorganics. Knowledge of the most adapted methodologies to solve an analytical challenge in a complex system.
Total number of hours: 14 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: A theoretical background on chemistry and analytical chemistry (M1 level) is required to attempt to this UE.
Teaching methods and activities: Lectures (CM)
Assessment: written exam (60%), project presentation (40%)
![]()
Course Title: Professional Skills
Course supervisor:
- S. Zrig
Description: This course encompasses: (i) a two-days workshop to learn how to write a résumé and a cover letter, (ii) a lecture and a practical session to discuss how to seek and plan an internship and (iii) two personal interviews with the Master 1 supervisors.
Keywords: Abstract, cover letter and internship search andpersonal interviews
Total number of hours: 6 + Workshop Number of ECTS: 1 Semester 1
Mandatory course ☒ Optional course ☐
Exact location: Université de Paris and Paris Rives Gauche (LAVOISIER building)
Prerequisites/skills needed: None
Teaching methods and activities: Lectures (CM), practical sessions (TD) and other : whorkshop
![]()
Course Title: Advanced computational project
Course supervisor:
- F. Barbault
Goals: This UE is a continuation of the « computational tutored project » UE with the more ambitious objective to let the students being able to manage and realize, alone, a computational project. After shorts lectures on the states of the art of computational modeling, each student will have to choose one subject and will be guided on how to manage it.
Description:
Introductive courses (8h): These lectures are intended to provide an introduction ot some of the techniques used in computational chemistry and to illustrate how these ones can be used to study physical, chemical and biological phenomena.
Tutored project (22 h): Each project will be realized under the supervision of a teacher and after validation of its feasibility. The project will be selected by the students according to their will. According to the number of people, similar projects might be gathered together. At the end of the session, a comprehensive report followed by an oral defense will be produced and used as evaluation for the validation of this UE.
Acquired skills: At the end of this UE the students will be able to: manage a computational project; undertake meaningful calculations according to experimental data; master molecular modeling results interpretations/discussions/critics; write and defend the computational results.
Key words: molecular modeling, molecular dynamics, structure-activity relationships, simulation of materials, quantum computations, nanosciences
Total number of hours: 30 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: Notions in physical chemistry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Report (50%) and oral defense (50%)
![]()
Course Title: Green chemistry : recent updates
Course supervisor:
- P. Busca
Goals: In this course, we will study the most recent advances in green chemistry, thanks to the Nature web site dedicated to this research axis.
Description:
Introductive courses (8h)
Four lectures are intended to provide students with basic principles of green chemistry.
Tutored project
Two sessions will be dedicated to teach the methodology for litterature search and critical analysis, as well as tips for written and oral presentation. Each pair of student will choose its own topic, and will be tutored to progressively be able to present their results in front of the class.
Acquired skills: bibliographic search and critical analysis
written and oral presentation
Key words: green chemistry
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: Basic principles of chemistry
Basic knowledge of green chemistry 12 rules
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: mid-term report (50%) and oral presentation(50%)
Course Title: Synthesis of bioactive compounds
Course supervisor:
- H. Dhimane
Goals:
- Mastering the main synthetic methods to access heteroaromatic compounds and their use as scaffolds for construction of biologically relevant compounds.
- Retrosynthetic analysis for multistep synthesis of bioactive compounds. Understanding the choice of the protecting groups and the chirality control.
Description:
- Part 1: Synthesis of the most common aromatic heterocycles [five- and six-membered heterocycles, and bicyclic heteroaromatics] according to the main, currently established strategies (ionic or radical cyclisation, pericyclic reactions, multicomponent condensations and transition metal-catalyzed reactions); the course will focus on the mechanistic and selectivity issues.
- Part 2: Multi-step synthesis:
Polyfunctionalized enantiopure building blocks with one or several stereogenic center(s) from chiral pool (carbohydrates, amino acids, terpenes and their derivatives)
Total synthesis of some relevant bioactive compounds (muricatacine, antibiotics, glycol- and peptido-mimetics)
Acquired skills: Deeper knowledge of the organic synthesis tools (named reactions and functional inter-conversions) for an efficient control of the selectivity issues (chemo-, region and stereoselectivity), enabling the student to design and perform efficient and reliable multi-step synthesis of targeted bioactive compounds.
Key words: Heterocycles, chiral pool, reactivity of heterocycles, protecting groups, multistep synthesis
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: Good knowledge of the reactivity and inter-conversion of main functional groups of organic chemistry.
Enough knowledge of the main methods for C-C bond formation.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Students will be assessed by a final (2 hours) written examination (100%).
Course Title: Modern spectroscopy for atmospheric and astrophysical applications
Course supervisor:
- F. Kwabia Tchana
Goals: The purpose of this course is to introduce the underlying spectroscopy linked to atmospheric and astrophysical studies. A special focus will be made on climate change and air pollution which are two key issues in current atmospheric research. Examples of observation of atmospheric key species by satellites and ground-based observatories will be given. The process of deducing mixing ratios from atmospheric spectra (retrieval) will be described and detailed.
Description:
Synchrotron radiation (SR) as a light source (6h CM + 3h TD):
- General mode of operation: electron storage rings, dipole and undulator beamlines
- Different spectral domains: IR, VUV,…examples of different scientific applications (condensed and gas phase examples)
Advanced laboratory spectroscopy in the THz and IR spectral domain for astrophysical and atmospheric applications
- Measurement of high quality spectroscopic data in the laboratory for the interpretation of observational data. Spectroscopic measurements at different temperatures and pressures.
- FTIR spectrometers, use of synchrotron radiation in the IR/THz domain, tunable diode laser spectroscopy in the IR/THz domain, cavity ring down spectroscopy (CRDS)
- Examples of the study of stable and unstable species of atmospheric and astrophysical interest
LiDAR: Light Detection And Ranging (6h CM + 3h TD):
Atmospheric and Astrophysical applications
- Structure of the Earth’s atmosphere
- Climate change and air pollution: key chemical and physical processes
- Atmospheric sounding: satellites and ground based observatories
- Chemistry in the interstellar medium and planetary atmospheres (including exoplanets).
- Spectroscopic observation: microwave and submillimeter telescopes, IR observation from satellites.
- Laboratory Astrophysics: measurement of high quality spectroscopic data for the interpretation of observational data. Spectroscopic measurements at different temperatures and pressures, examples of the study of species of atmospheric and astrophysical interest.
Practical work in the form of mini-projects
- Visit to French Synchrotron SOLEIL
- Analysis of an atmospheric spectra
Acquired skills: To know and understand observation techniques using spectroscopic methods in universe sciences, initiation to atmospheric spectrum analysis and inversion methods.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: Basic knowledge of L3/M1 level spectroscopy in chemistry or physics. Physico-chemistry of the atmosphere and astrophysics.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final exam (100%) : Project (report + presentation)
Course Title: Bio-Organic Chemistry
Course supervisor:
- O. Reinaud
Goals: Discovery of the tools developped by Nature for redox signaling and organic co-factors at the molecular level. Basic associated mechanism. Oxidative processes with sulfur compounds.
Description:
1. Electron transfer in the respiratory chain
2. Oxidative modifications on cysteines and redox signaling:
Oxygen and nitrogen reactive species (ROS/RNS): formation and reactivity.
Biological chemistry of sulfenic acids and nitrosothiols.
3. Biological Chemistry of organic co-factors: PLP; TPP, SAM
4. Allosteric Regulation by small molecules
Acquired skills: Understanding the concepts and molecular chemistry involved in redox signaling on one hand and of organic cofactor on the other hand
Key words: Enzyme– regulation- co-factor-sulfur
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☐ Optional course ☒
Prerequisites/skills needed: Bases in organic chemistry and molecular biochemistry
Teaching methods and activities: Lectures (CM),
Assessment: Oral exam (100 %)
![]()
Course Title: Advanced Electrochemistry and Applications
Course supervisor:
- M. Robert
Goals: L’objectif de cette UE est, à partir de solides fondamentaux en électrochimie moléculaire, de montrer les principes et applications de la catalyse en électrochimie, en développant en particulier l’étude de l’activation de molécules clefs dans l’émergence actuelle des énergies renouvelables mais aussi en étudiants les biomolécules redox. Concernant l’énergie, deux aspects seront envisagés : l’électrocatalyse et la catalyse moléculaire. Dans le second cas, l’objectif sera notamment que les étudiants acquièrent les concepts permettant l’analyse, via les méthodes électrochimiques, des mécanismes mis en jeu. Le cours sera ensuite poursuivi par une introduction approfondie aux nano-matériaux catalytiques pour l’énergie (procédés de mise en forme multi échelles des matériaux et l’évolution de leurs propriétés en fonction de la taille, de la forme et du volume des objets). Cette UE s’appuie sur l’enseignement de tronc commun comportant les bases de l’électrochimie.
Description:
• Méthodes avancées en électrochimie moléculaire (4h)
• Catalyse moléculaire des réactions électrochimiques (4h)
• Application de la catalyse moléculaire à l’activation de petites molécules : réduction des protons, oxydation de l’eau, réduction du CO2, réduction du dioxygène. (4h)
• Voltamétrie cyclique des enzymes redox (4h)
• Electrocatalyse : principes et applications (4h)
• Nanomatériaux et catalyse pour l’énergie (4h): Structuration des surfaces, Phénomènes de transport ioniques (H+, O2-, etc.) et électroniques, Réactivité et catalyse
Acquired skills: concepts de la catalyse moléculaire et bio-moléculaire électrochimique et de ses aspects mécanistiques, principes de l’électrocatalyse et ses applications, connaissances générales des matériaux catalytiques nano-structurés pour l’énergie.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Electrochimie de M1 ou équivalent, base de chimie moléculaire, bases de la réactivité des surfaces
Assessment: Contrôle terminal (examen et présentation d’article)
Course Title: Collaborative research project Part II
Course supervisor:
- V. Noel
Goals: The aim is to introduce Master 2 students to project research and to strengthen their soft skills related to collaborative work.
Description:
Ce cours s’inscrit dans la continuité de l’UE “Research Project 1”.
Students will be divided into groups of 3 to 4 people constituting a team. Several teams will be associated to form a consortium whose mission is to build a collaborative research project of a European type (FET-OPEN or RIA). Each consortium will have to defend its research project in front of a panel (students + teachers), i.e. to justify the project structure, budget, expected timetable as well as the scientific and technological proposed approaches.
Acquired skills: Communication skills such as articulating ideas, clarity and concision in argues description, public speaking…
Total number of hours: 24 Number of ECTS: 3 Semester 4
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Research Project 1
Assessment: Examen Final (Oral Presentation) -100 %
![]()
Course Title: Analyse de Surfaces
Course supervisor:
- L. Sicard
Goals: Les objectifs de cette UE sont multiples. Il s’agit d’abord de décrire les principes de fonctionnement des techniques d’analyses physico-chimiques à base de rayons X sélectionnées afin que les étudiants puissent : i) identifier les applications potentielles liées aux spécificités de chaque technique ; ii) être capables de choisir les différentes techniques de caractérisation complémentaires à mettre éventuellement en œuvre pour une analyse donnée. Enfin, les étudiants seront formés vis-à-vis de l’interprétation des mesures ou des images obtenues par les différentes techniques d’analyse sélectionnées dans le cadre de cet enseignement.
Description:
Spectroscopie photo-électronique X (XPS), Fluorescence X (XRF), Diffraction des rayons X (XRD), Diffusion des rayons X aux petits angles (SAXS)
Acquired skills: Les étudiants formés seront à même d’identifier les techniques d’analyses pertinentes à mettre en œuvre pour l’étude d’un matériau donné mais seront aussi capables d’exploiter les données obtenues dans le cadre de ces analyses.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: M1
Assessment: Contrôle terminal (70%) TP (30%)
![]()
Course Title: Imagerie de Surfaces
Course supervisor:
- N. Battaglini
Goals: Les objectifs de cette UE sont multiples. Il s’agit d’abord de décrire les principes de fonctionnement des techniques d’analyses physico-chimiques de surface sélectionnées afin que les étudiants puissent : i) identifier les applications potentielles liées aux spécificités de chaque technique ; ii) choisir les différentes techniques de caractérisation complémentaires à mettre éventuellement en œuvre pour une analyse donnée. Enfin, les étudiants seront formés vis-à-vis du traitement et de l’interprétation des images et des données obtenues par les différentes techniques d’analyse retenues dans le cadre de cet enseignement.
Description:
Microscopie électronique en transmission (TEM), Microscopie à sonde locale (SPM), Microscopie électrochimique à balayage (SECM)
Acquired skills: Les étudiants formés seront à même d’identifier les techniques d’analyses pertinentes à mettre en œuvre pour l’étude de surfaces et de nanomatériaux donnés mais seront aussi capables d’exploiter les données obtenues dans le cadre de ces analyses.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: M1
Assessment: Contrôle terminal (70%) TP (30%)
![]()
Course Title: Chemiometrics in heterogeneous and complex systems
Course supervisor:
- Rémi Losno
Goals:
Performing analyses is a multi-step process, from sampling to the final result. A random variability is added at each step and is evaluated to assess the quality of the final results. Analytical chemistry of complex systems including Environment produces lots of data in short periods to take into account the time evolution and the spatial heterogeneity of the systems. Understand the meaning of the data stream is a challenge but necessary to understand the fate and behaviour of the investigated system. Most of the useful information can be hidden in the data flow and the objective of this course is to learn how to extract the maximum information with the minimum of work. Computation tools are available and will be used at the user level.
Program:
1) Data analyses (8h, lectures):
- Review of statistical methods used for measurements and time series processing
- Accuracy. Errors and uncertainties. Error propagations.
- Analyses certification. Instrument stability check.
- Compositional data handling and analysis (CoDa).
2) Sampling strategy in an heterogeneous system: space and time heterogeneity (4h)
3) Computation training using R and spreadsheets (8 h Practical work)
Key words: statistics, uncertainties, method validation, chemical data processing
Total number of hours: 20 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: A theoretical background on chemistry and analytical chemistry (M1 level) is required to attend this UE.
Teaching methods and activities: Lectures (CM), Lab sessions (TP), practical sessions (TD)
Assessment: Written examination (60%), practical work and reports (40%)
![]()
Course Title: Environmental pollution, health and the Biosphere
Course supervisor:
Title: Dr.
First Name: Etienne
LAST NAME: Blanc
Description:
This course at the interphase between biology and chemistry, aims to provide an overview of the interactions taking place between chemicals and living organisms. This UE is thus an introduction to the study of the impact of anthropogenic and natural pollution on living organisms, including the human body. It will replace molecules and their characteristics in a more global context of interaction with the environment. In particular, the impact and distribution of chemicals between the environmental media biota, air, water, and soil/sediment will be discussed based on their physical and chemical properties The fate, the behavior of pollution in the different environment (air, water, soil) will be studied. Particularly the source, fate and impact of some pollutants (as pesticides, microplastics…) will be studied. Some examples of human impact on air or water resources will also be presented as smog, ozone layer, global climate change, ocean acidification … also part of the courses will address the use of microorganisms in the context of bioremediation for the removal of organic pollutants
Program (7 weeks + postponed exam):
- A. Gratien: Fate and behavior of pollutants in the environment / global impact of pollutant on the biosphere: 7h
- E. Blanc: Pollution and human health: CM 4h + 1,5h TD (article analysis)
- E. van Hullebusch: Introduction to bioremediation approaches for organic pollutants: 4h
Learning objectives : After completing this UE, the students will be able to:
- Have a good understanding the impact of chemicals on the environment from the biological view point
- Be able to select tools to measure chemical health or biological impacts
- To understand and interpret concept and data reported in scientific publication focusing on the processes taking place at the chemistry / biology interface
Total number of hours: 16.5 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Exact location: UFR Chimie, Campus Grand Moulin
Assessment: Final exam (60%), Research Presentation assignment (40%)
![]()
Course Title: Quantitative organic analyses
Course supervisor:
Title: Professor
First Name: Pascal
LAST NAME: Houzé
Goals:
This course aims at offering an overview of the analysis and quantification of small molecules such as drugs or pollutants, using NMR and coupled separation methods.
Description:
Basics of quantitativity in NMR:
- Application to solution NMR : 1D and 2D analyses
- Application to solid-state NMR : CPMAS / DNP, …
Chromatography and coupled methods:
- General consideration in chromatography: instrumentation, physical quantities, principles of separation, …
- Introduction to coupled methods : GC/MS, LC/MS
- Applications to the study of small molecules in biological samples.
Acquired skills: The students will acquire a dual skill in therapeutic nanoengineering and diagnostic.
Key words: RMN, Chromatography, Mass spectrometry, quantification
Total number of hours: 18 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Exact location: Université Paris Cité, campus des grands moulins
Prerequisites/skills needed: Theoretical background on NMR, experience in the use of 1D and 2D basic experiments. Basic background on separation methods and mass spectrometry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
![]()
Course Title: Analyse of elements, minerals and isotopes
Course supervisor:
- Didier Jézéquel
- Yann Sivry
- Sophie Nowak
- Rémi Losno
Goals:
The purpose of this course is to provide an overview of i) the main issues and solutions relatives to analytical measurement on complex matrix; ii) the specificity of techniques used for such measurements; iii) the most recent developments on this subject.
Description:
The overall goal of this UE is to present the main issues relative to analytical measurement on environmental and complex systems, from the sampling strategy to the use of analytical techniques dedicated to such systems in the current scientific state of the art.
This objective will be reached thanks to the development, during the lectures and tutorials, of specific themes:
- Analytical techniques using spectrometry (AAS, ICP-AES, ICP-MS) (YS, 2h CM + 2h TD);
- Chromatography, electrochemistry and coupled devices (DJ, 2 h CM + 2h TD);
- X-Rays diffraction (XRD),(SN, 2h CM);
- Analytical techniques using spectrometry (XRF) (RL, 2h CM);
- Isotopic determinations as a tool to decipher complex systems (YS, 2 h CM);
- Environmental monitoring (DJ, 2h CM);
- On-site visit of ICP-MS and tutorial on data treatment (RL, 2h).
Both analytical development challenges and applied case studies will be presented in this course.
Acquired skills: Advanced method and technics for elemental, isotopic and molecular inorganic analyses in heterogneous and variable systems.
Total number of hours: – Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: A theoretical background on chemistry and analytical chemistry (M1 level) is required to attempt to this UE.
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: Oral presentation and examination (100%). Each student will have to give a 15 min talk dealing with either a case study of complex system analysis, an analytical method specifically dedicated to environment and complex system analyses or a comparison of data obtained with various analytical techniques. The student ability to criticize the choice of the techniques and the analytical precisions will be taken into account. This presentation will be followed by an oral examination of 15 min.
![]()
Course Title: Environment: structure and processes
Course supervisor:
- M. Benedetti
Goals:
The purpose of this course is to provide an understanding of the fundamental geochemical processes that govern the chemical composition of natural soils, waters and air and the environmental fate of chemical species in natural waters.Description:
Thermodynamics, kinetics and transport processes regulating the chemistry of air, soil, surface and groundwater in natural and polluted environments, with particular emphasis on explaining the aqueous concentrations of chemical species and controlling geochemical factors in the hydrosphere.
Lectures list :
• Complexation Reactions (MB, 2h+ 2h TD)
• Chemical Weathering (MB, 2h)
• Sorption and Desorption (YS, 2h)
• Modeling interface processes (MB, 2h cours + 2h TD)
• Redox Chemistry (DJ, 2h cours + 2h TD)
• Kinetics and transport (DJ, 2h)
Presentations: Each student is required to give two 15 min talks to the class on a specific research topic related to the material covered in class. The talks will be based on a literature search involving journal papers.
Tutorials will require the use of the geochemistry program Visual MINTEQ. A download copy (free) is available at http://www.lwr.kth.se/English/OurSoftware/vminteq/ (this program is designed to operate in Windows).
Acquired skills: Knowledge of chemical evolution in the environment and in heterogeneous media.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: A theoretical background on chemistry (M1 level) is required to attempt to this UE.
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: Final exam (60%), Research Presentations (40%)
![]()
Course Title: Chemical Engineering of nanoparticles for therapy
Course supervisor:
- M. Hémadi
Goals:
- This course deals with innovation approaches on nanomedecine in the fields of therapy, diagnosis and theranostic. Nanomedicine is at the forefront of modern healthcare. Nanoparticles offer a new platform for drug delivery that can extend the “patent life” of drugs, but also greatly increase the targeting and effectiveness of therapy. They can enhance most of the medical imaging modalities, and in some cases offer a combined diagnostic and therapy, now called “theranostics”. This course aims to provide students with the necessary training to be able to understand the principles of nanotechnology and its application in medical research and clinical practice.
- The practical course aims to broaden the knowledge and skills of the students in the areas of nanoparticle synthesis, characterization and processing in biomedical applications.
Description:
1. Cours (16h) ;
● Drug release and Diagnostic
– Nanomedecine – introduction (biodistribution, targeting, clearance);
– Drug release ;
– Diagnostic;
● Therapy – thermotherapy
– Interaction between nanoparticles and cells
– Hyperthermia
2. Expériences (2 TP de 4h) ;
① Synthesis of nanoparticles and characterization ;
② Biomedical applications : fonctionnalization by biomolecules and thermotherapy
Acquired skills: The students will acquire a dual skill in therapeutic nanoengineering and diagnostic.
Key words: nanomedecine, drug delivery, thermotherapy, diagnostics
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basic notions of molecular and material chemistry and biology
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: 1 exam (relating to the theoretical and experimental parts of the course) 60% + TP 20% + bibliographic presentation 20%
Course Title: Nanomatériaux avancés pour l’optique et le magnétisme
Course supervisor:
- J-Y. Piquemal
Goals:
L’objectif de cette UE est d’une part de permettre aux étudiants d’acquérir une connaissance fondamentale et une compréhension fine des méthodes physiques et chimiques permettant de concevoir des structures nanométriques. L’accent sera mis en particulier sur les méthodes. D’autre part, il s’agira de montrer le lien existant entre la dimension et le degré d’organisation des édifices et les propriétés physico-chimiques résultantes. Une attention particulière sera portée sur la modulation des propriétés optiques et magnétiques à l’échelle nanométrique.
Description:
1) Elaboration de nano-structures (1 ECTS)
• Panorama des méthodes de synthèse par voie physique et voie chimique
• Méthodes d’élaboration avancées par voie chimique.
2) Nano-optique (1 ECTS)
• Propriétés optiques de nano-structures de métaux nobles : notion de constante diélectrique, plasmon de surface localisé ;
• Application aux spectroscopies à haute sensibilité : diffusion Raman exaltée de surface, spectroscopie de fluorescence exaltée ;
• Plasmonique moléculaire : capteurs moléculaires, nano-structures hybrides actives.
3) Nano-magnétisme (1 ECTS)
• Les contributions énergétiques : Energie d’échange, énergie magnétocristalline, énergie magnétostatique, énergie de surface et d’interface
• Effets de taille, dimensions caractéristiques : configurations magnétiques, tailles critiques, superparamagnétisme, effets de forme.
• Mécanismes de retournement de l’aimantation (parois, curling, rotation uniforme, modèle de Stoner-Wolfahrt) – Propriétés dynamiques de l’aimantation.
• Relation structure, dimension, propriétés
• Applications : Magnétorésistance géante, enregistrement magnétique haute densité, absorption hyperfréquences, …
Acquired skills: élaboration de nano-structures, propriétés optiques et magnétiques de nanostructures métalliques, plasmonique moléculaire, spectroscopies exaltées à haute sensibilité , …
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: physico-chimie des surfaces et interfaces, électromagnétisme, spectroscopies (absorption, fluorescence, Raman)
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: Examen terminal (100%)
![]()
Course Title: Molecular Electronics, Organic and Printed
Course supervisor:
- J-C. Lacroix
Goals:
Nowadays, the microelectronic industry is facing increasing and strong technological/market demands pushing towards devices miniaturisation, fabrication costs reduction and the integration of novel functionalities into electronic devices (for instance, flexibility). Such needs may be not completely satisfied by traditional, silicon-based electronics but they require the development of new concepts where crystalline, inorganic semiconductors are replaced by single molecules (molecular electronics) or thin films of organic, conjugated polymers (organic electronics) and traditional cleanroom-based fabrication techniques are replaced or complemented by techniques that use solution-processable materials deposited at ambient conditions (printed electronics). The goal of this UE is to introduce the student to the most important concepts of these new, emerging fields.
Description:
1) Nanoelectronics, molecular electronics
2) Printed electronics and Thin Film fabrications processes
– Thin films deposition and characterisation techniques (thermal evaporation, CVD, spincoating,…)
– Printed electronics: printing techniques, inks and substrates
– Organic thin film transistors
Acquired skills: At the end of this course the student will be able to identify the different techniques available for the deposition of a thin film. He/she will be also able to choose the most appropriate techniques for the characterisation of the thin film physical properties (thickness, roughness, adhesion to the substrate…). The student will also acquire a basic knowledge of the field of printed electronics. He/she will be also able to describe the most important types of organic thin film transistors.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: M1
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: Contrôle terminal
![]()
Course Title: Redox bio-inorganic chemistry
Course supervisor:
- O. Reinaud
Goals: Discovery of the structures, mechanisms and reactivity of major classes of metallo-enzymes (Fe, Cu) involved in redox processes
Activation of small molecules (H2O2, O2), Biomimetic systems
Description:
I. For each class, the student will have to prepare an oral presentation on a metallo-enzyme considered as a prototype of a specific family, mainly oxidase or oxygenase. The major types of related Fe and Cu enzymes will be covered, with a major insight into the catalytic mechanism at the active site associated to key spectroscopic studies.
II. Articles with questions related to biomimetic systems aimed at modeling the enzyme active site will be given and the students will have to answer these questions (related to the research methodology, the validity of the results, the interpretation etc…). A discussion will follow.
Acquired skills: Knowledge and understanding at the molecular level of redox reaction catalyzed by metallo-enzymes and design of biomimetic systems
Be able to propose a new experiment for gaining new insights into the catalytic mechanism
Be able to propose and write a mechanism for an unknown enzyme
Be critic vs. proposed mechanisms
Key words: dioxygen activation – copper -iron –enzymes-model complexes
Total number of hours: >24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basic knowledge in biological chemistry and bio-coordination (amino-acid residues).
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final exam (50%) + continuous monitoring
(participation to the discussion, oral presentations 50%)
Course Title: Biomolecular Diagnostic and biosensing
Course supervisor:
- F. Mavré
Goals: The main objective is to understand how chemists, biomolecular chemists and physical-chemists may develop strategies in order to help the quantification of relevant molecules such as nucleic acids, proteins, small organic or inorganic molecules in a complex biological sample.
Description:
How can a diagnostic test be selective? How can it be sensitive? What are the most relevant format? The main challenge in molecular diagnostic is to reveal the selective reactivity of the targeted molecule with a sensing unit (an immunoreaction, a DNA hybridization, an enzyme/substrate reaction…) and then translate it into an appropriate readout (electrical signal, optical signal …). This can either involve homogenous (bio)-chemical reactions or more complex interfacial chemistry. Separation, amplification and calibration steps may be also required. This course will describe the physico-chemical basis of gold standards bioanalytical techniques (immunoassays; nucleic acids assays; enzyme-based assays; biosensors) and explore the newest concepts in the field.
The course will be divided in the following five sequences:
• General concepts of biomolecular diagnostics (8hrs)
• Kinetics and thermodynamics of biomolecular recognition on surfaces (4hrs)
• Surface modification of sensing interfaces (2hrs)
• Strategies for signal amplification (6hrs)
• Enzymatic biosensors (4hrs)
Acquired skills: The students will be able to understand principles lying under the detection of a target biomolecule of interest in a complex media, and select the most adapted bio-analytical tools to address specific issues.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basic knowledge in biological chemistry and bio-coordination (amino-acid residues).
Teaching methods and activities: Lectures (CM),
Assessment: Final Exam (100%).
![]()
Course Title: Chemical Engineering of nanoparticles for therapy
Course supervisor:
- C. Mangeney
Goals:
- This course deals with innovation approaches on nanomedecine in the fields of therapy, diagnosis and theranostic. Nanomedicine is at the forefront of modern healthcare. Nanoparticles offer a new platform for drug delivery that can extend the “patent life” of drugs, but also greatly increase the targeting and effectiveness of therapy. They can enhance most of the medical imaging modalities, and in some cases offer a combined diagnostic and therapy, now called “theranostics”. This course aims to provide students with the necessary training to be able to understand the principles of nanotechnology and its application in medical research and clinical practice.
- The practical course aims to broaden the knowledge and skills of the students in the areas of nanoparticle synthesis, characterization and processing in biomedical applications.
Description:
1. Cours (16h) ;
● Drug release and Diagnostic
– Nanomedecine – introduction (biodistribution, targeting, clearance);
– Drug release ;
– Diagnostic;
● Therapy – thermotherapy
– Interaction between nanoparticles and cells
– Hyperthermia
2. Expériences (2 TP de 4h) ;
① Synthesis of nanoparticles and characterization ;
② Biomedical applications : fonctionnalization by biomolecules and thermotherapy
Acquired skills: The students will acquire a dual skill in therapeutic nanoengineering and diagnostic.
Key words: nanomedecine, drug delivery, thermotherapy, diagnostics
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basic notions of molecular and material chemistry and biology
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: 1 exam (relating to the theoretical and experimental parts of the course) 60% + TP 20% + bibliographic presentation 20%
![]()
Course Title: Molecular and Nanoprobes for imaging
Course supervisor:
- C. Mangeney
Goals: The UE Molecular and nano-probes for imaging brings the chemistry expertise of the modern imaging probes and nanomedecine used and developed in all medical imagings, especially molecular and functional imaging using chemical contrast agents (properties, interaction,…) to be able to handle the acquisition of the images in all modalities, and use the current and latest imaging probes and nanotechnology for the diagnosis and the development of innovative therapy in preclinics to clinics. Newest technological developments of the bioimaging probes for diagnosis and therapy, from in vitro to in vivo, from small animal to human are presented.
Description:
1. Cours (16h) ;
● Introduction on imaging contrast agents – 6h Souad Ammar
● MRI – 4h Bich-Thuy
– MRI contrast agents;
– Diagnostic;
● Optical imaging, Echography, Photoacoustic imaging 6h Florence Gazeau – Cyrille Richard
– Ultrasound probes
– Fluorescence
– Photoacoustic probes
2. Expériences (2 TP de 4h)
① Magnetic resonance Imaging ;
② Optical imaging or ultrasound or photoacoustic
Acquired skills: Understanding of imaging agent properties for all bioimaging modalities, familiarization with advanced in-vivo imaging techniques
Key words: Bioimaging, Contrast agents, Molecular probes, Nanoprobes
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basis in molecular and material chemistry
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: Terminal exam 50% and Oral presentation 50%
![]()
Course Title: NMR of Small Molecules – structure and dynamics
Course supervisor:
- N. Giraud
Goals: Understanding the fundamental principles of NMR spectroscopy, and its application to the structural and dynamics analysis of samples in the biomedical field.
Description: Liouville – Von Neumann equation – Spin interactions – Introduction of product operator formalism
Principle of NMR experiments: pulse sequence, spectrometer, quadrature detection, phase…
Spin evolutions: interaction with an rf field, precession, relaxation, spin echoes
Multi-dimensional NMR: detection scheme, phase cycling, pulsed field gradients
Homo- and heteronuclear correlation experiments. Description of pulse sequences using product operator formalism (COSY, HSQC, INEPT…)
Relaxation (T1, T2, nOe effect), application to structural and dynamic analysis of biomolécules. NOESY and ROESY experiments
Dynamic processes: chemical exchange, diffusion
Hyperpolarisation (DNP …)
High-resolution NMR : higher, faster, better
Acquired skills: Students will be able to elaborate a theoretical description of the evolution of a spin system during a pulse sequence. They will learn how to apply these theoretical tools to the analysis of an unknown sample. They will learn how to choose and implement the best experiment for the structural or dynamic issued that is addressed. They will understand modern approaches that have been implemented over the last decade to improve sensitivity, resolution, and the rate of acquisition of NMR analyses.
Key words: NMR, pulse sequences, multi-dimensional NMR, correlation spectra, nuclear spin relaxation, chemical exchange, diffusion, hyperpolarisation.
Total number of hours:30 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: NMR Lecture from UE8 : Principle of 2D NMR, description of main pulse sequences, bases of nuclear spin relaxation (T1, T2, effet nOe), chemical exchange in RMN.
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP), autres : projet personnel et/ou travail en autonomie
Assessment: Examen final + soutenance projets
![]()
Course Title: Advances for Biomolecule modifications
Course supervisor:
- M. Etheve-Quelquejeu
Goals: The aim of this course is to provide an understanding of the chemical strategies to modify biomolecules, in particular, proteins and nucleic acids.
This will include chemistry of amino acids, nucleosides and nucleotides.
At the end of this course, students will be able to play with protecting groups and orthogonal reactions and to use synthetic and enzymatic methodologies for post-functionalization and bioconjugaison of biomolecules.
Some applications will be also discussed, such as the used of modified biomolecules and conjugates in the field of new therapeutical strategies, for targeted delivery, for the production of DNA-encoded libraries and aptamers.
Description: Chemistry of amino acids, proteins, nucleosides and nucleotides.
Post-functionalization methodologies: Click chemistry, Native Chemical Ligation, Staudinger ligation, enzymatic reactions. Combinatorial chemistry as selex for the production of DNA-encoded libraries and synthesis of aptamers.
Acquired skills: Organic chemistry and post-functionalization of biomolecules apply to peptides, proteins and nucleic acids (DNA and RNA). Solid phase synthesis. Combinatorial chemistry. Applications in gene therapy and biosensing and design of probes for biological studies.
Key words: Peptides, nucleotides, proteins, nucleics acids, solid phase synthesis, click chemistry, native chemical ligation, bioconjugaison, post-functionalization, selex, aptamers, ribozymes, anti-sens strategies.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Organic chemistry in Master one
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
![]()
![]()
Course Title: Interaction with Biomolecules 1
Course supervisor:
- N. Giraud
Goals:
Mass Spectrometry : Students will learn how to choose the adequate ionisation and analysis method that are the most suitable for addressing a particular question, in the field of proteins, « small molecules », non-covalent protein complexes or RNA.
Biomolecular NMR : Students will be able to read a publication addressing a scientific question involving proteins and RNA using NMR spectroscopy. They will notably learn how to determine if the protein is folded, if macromolecules interact with each other, and they will be able to propose the best isotopic labeling and select the most pertinent NMR experiments to answer the question at hand.
Isothermal Titration Calorimetry : Students will learn how to determine the thermodynamic constants that are involved in the interaction process between biomolecules.
Acquired skills:
Mass Spectrometry : Students will acquire an extended knowledge of the different ionisation and analysis methods in mass spectrometry (MS and MS/MS), in order to choose the most pertinent one for addressing the study of a given molecular system.
Biomolecular NMR : Students will be able to find the right information and to understand scientific publications presenting projects that were carried out using NMR as a technique for the analysis of RNA/protein interactions.
Isothermal Titration Calorimetry : Students will know how to determine an affinity constant, and will be able to interpret their enthalpic and entropic contributions.
Key words: mass spectrometry, structural biology for protein and protein-ligand complexes analysis, structural chemistry
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed:
Mass Spectrometry : fundamentals of organic and biological chemistry, in particular the structure of (macro)molecules, their reactivity (acido-basic, nucleophile, electrophile). Analytical chemistry (chromatography), physical chemistry (electronic and vibrational energies)
Bio molecular NMR : Basic knowledge of proteins (primary, secondary, tertiary) and nucleic acids
Isothermal Titration Calorimetry : basics of thermodynamics
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: 100% Final examination
![]()
Course Title: Interaction with Biomolecules 2
Course supervisor:
- N. Félidj
Goals: Interactions between biomolecules (protein, DNA, etc.) play a fundamental role in signalling pathways that regulate numerous cell functions. Study of these interactions makes it possible to understand the biological mechanisms and the physiopathology of many human diseases (cancer, neuro-degenerative illnesses). Their understanding thus offers possibilities for therapeutic intervention, by developping interface inhibitors, often more specific that inhibitors of enzymatic activity.
After defining the characteristics of interactions between biomolecules (thermodynamic, kinetic…) by presenting the structural and functional properties of these interfaces, we shall study physicochemical and analytical methods and techniques to detect these interactions, such as: Surface Plasmon Resonance imaging (SPRi), Fluorescence, Infrared (IR) et Circular Dichroism (CD).
We will illustrate the different parts of the lecture with various examples, possibly in the context of tutorials and tutorial projects.
Description:
- SPR technique: Total internal reflexion, surface plasmon polariton, instrumentation, applications in biochemistry (SPRi)
- Infrared (IR): vibrational spectroscopy
- Fluorescence emission spectroscopy, thermodynamic and kinetic aspects of an interaction (enhancement, quenching, FRAP, FRET, FLIM, etc.). Stern-Volmer law. Imaging. Confocal microscopy.
- IR Technique: notions of vibrational spectroscopy, study of the conformation of biomolecules and the nature of chemical bonds.
- Circular Dichroism: notions of absorption spectroscopy, evolution of the overall structure of biomolecules, as well as conformational changes upon interaction.
Key words: Surface plasmon resonance, IR, biosensors, fluorescence.
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed:
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final examination: 100%
Course Title: Case Studies
Course supervisor:
- B. Colasson
Goals: A specialized class on a particular topic of broad interest in line with supramolecular aspects for the students of all the “itinéraires”.
4 topics emerged from the 4 “itinéraires” (to be decided) and 2 topics will be presented by a lecturer from another university.
For each lecture, the teacher gives in advance a review and a research article to read, together with a questionnaire relative to the research article. The students have to send their written answers to the teacher 3 day before the lecture.
During the class, each professor gives first a 2h lecture on a specific topic. This general presentation is followed by an analysis of the research article and discussion with students.
Acquired skills: Knowledge of important areas of research using the principles of supramolecular chemistry for developing functions aimed at applications.
Be able to extract the major information out of a review article on a new topic
Be able to do a critical reading on a research article related to a new topic and getting the important facts and perspectives
Total number of hours: 18-21 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basic concepts in supramolecular chemistry M1 level.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: 100 % Final written exam
Course Title: Collaborative research project – Part 1
Course supervisor:
- V. Noel
Goals: The aim is to introduce Master 2 students to research projects and to strengthen their soft skills related to collaborative work.
Description:
This course will be divided into two parts. The first part will be taught under a classical form (lectures, seminars) allowing students to acquire the necessary knowledge to successfully carry out the second part, were they will be asked to work independently to build their own collaborative research project.
Part 1: In an initial stage, the main research funding agencies (public and private) will be presented; the structure and management of collaborative research projects (timetables, milestones, deliverables…) will be also described. Several scientific experts will give seminars allowing grasping the contemporary trends and stakes of the discipline (chemistry and big data, the chemical industry 2.0, the chemistry at the interfaces…).
Part 2: In a second phase, students will be divided into groups of 3 to 4 people constituting a team. Several teams will be associated to form a consortium whose mission is to build a collaborative research project of a European type (FET-OPEN or RIA). Each consortium will have to structure, budget and justify its project using the planning tools and the ad-hoc methods.
Acquired skills:
- Analysis and summary skills of the scientific production in a given field (publications, patents)
- Ability to develop a creative approach to solve a scientific problem
- Team building, Integration into a working team and ability to be a source of propositions
- Capacity to present the results under different forms (oral presentations, written reports…)
Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: M1
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment:
Course Title: Organic reactivity 1
Course supervisor:
- G. Prestat
Goals: Mastery of catalytic and stoechiometric organometallic chemistry for synthesis.
Description:
Stoichiometric organometallic chemistry
Direct metalation, halogen-metal exchange, oxidative additions
Transtion-metal catalysis
C-C and C-N bond formation, C-H activation.
Acquired skills: Rules and methods for selective activation of bonds or functional groups.
Key words: Organometallic chemistry, transition-metal catalysis
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Mastery of basic knowledge and concepts in inorganic and organic chemistry acquired during M1 chemistry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final written exam 100%
![]()
Course Title: Organic reactivity 2
Course supervisor:
- G. Prestat
Goals: Mastery of asymmetric synthesis and radical organic synthesis.
Description:
Asymmetric synthesis
Sterechemistry and biological properties, conforlational analysis, dynamic stereochemistry, tranfer of chirality, chiral auxilliaries, TM-Cat, organocatalysis, dykat, desymmetrizations
Radical Chemistry
Radical initiator – Nucleophilic vs electrophilic radical – Cyclisation – Baldwin’rules – Keck – Reduction– Radical cascade – stereo-control.
Acquired skills: Rules and methods for enantioselective synthesis and radical based organic synthess.
Key words: Asymmetric synthesis, asymmetric catalysis, radical chemistry
Total number of hours: 24 Number of ECTS: 3 Semester 3
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Mastery of basic knowledge and concepts in inorganic and organic chemistry acquired during M1 chemistry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final written exam 100%
![]()
Course Title: Vibrational and optical spectroscopies
Course supervisor:
- C. Mangeney
Goals: This course deals with advanced optical spectroscopic techniques and nanospectroscopy. The objectives are to provide students with (i) a comprehensive knowledge in modern optical spectroscopic techniques used in research laboratories of universities and research centers; (ii) theoretical skills to be able to analyze Raman, Infrared, UV-visible and fluorescence spectra and (ii) experimental practice in nanoscience and nanospectroscopy.
Description:
1. Course (16h – 8 sessions of 2h) ;
– UV-visible and fluorescence spectroscopies;
– Infrared and Raman Spectroscopies;
– Nanospectroscopies.
2. Experimental activities (3 TP de 4h) ;
① Plasmonic nanoparticles: synthesis and optical properties – Groupe de 10 étudiants
Notions : – Synthesis of nanoparticles (nucleation and growth);
– Colloidal chemistry
② Detection of trace molecules by Fluorescence/Raman spectroscopies – Groupe de 6 étudiants
Notions : – Quantum yields and lifetimes of excited states;
– Enhanced spectroscopies, Surface-enhanced Raman spectroscopy
③ UV-visible spectroscopy and nano-optics – Groupe de 10 étudiants
Notions : – Optical properties at the nanometer scale ;
– Detection of molecules, sensors, colloidal stability and functionalization
Acquired skills: theoretical skills and knowledge in optical spectroscopy techniques and in nanospectroscopy
Key words: vibrational and optical spectroscopy, nanospectroscopy, fluorescence, Infrared, Raman, surface-enhanced Raman spectroscopy
Total number of hours: 28 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basic knowledge in spectroscopy
Teaching methods and activities: Lectures (CM), Lab sessions (TP)
Assessment: 1 examination (on the theoretical and practical parts) 60% + Practical activities 20% + poster session 20%
![]()
Course Title: Tutored project
Course supervisor:
- F. Berhal & S.Zrig
Description:
This course addresses numerous research topics in chemistry (medicine, materials, catalysis, energy…) and aims to introduce students to the management of a research project. The training sequence encompasses (i) the bibliographic study, (ii) the design and (iii) the development of an experimental protocol and the summary of the results. Targeted skills: bibliographic search tools; redaction of scientific articles; teamwork; interdisciplinary; project management.
Key words: Organic synthesis, organometallic synthesis, physicochemical characterization
Total number of hours: 26 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: First semester of the Master in Chemistry and Bachelor in Chemistry: Organic synthesis, physicochemistry, analytical chemistry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Exact location: Grands Moulins (LAVOISIER building)
![]()
![]()
Course Title: Redox metallo-enzymes
Course supervisor:
- O. Reinaud
Goals: The aim of this course is to discover the chemical principles governing redox reactivity by studying biological systems involved in electron transfer and oxygen metabolism. The chemical roles of the main organic and metallic cofactors (Fe, Cu) and their interactions will be discussed through the comparative presentation of metalloproteins and their chemical models. The focus is on studying mechanisms at the molecular level.
Description:
I. Tools for studying reaction mechanisms
– Thermodynamic and kinetic isotopic effects, tunnel effect
– Hammett equation
– Application to the study of reaction mechanisms
Réactions redox dans les systèmes biologiques non héminiques
II. A case study : Galactose oxidase and model compounds
A remarkable example of synergy between biology and chemistry
From the discovering of the mechanism at the active site to efficient catalysis with bio-mimetic metal complexes and /bio-inspired systems.
III. Tutored group working on redox metalloenzymes (Fe et Cu) and models
Oral présentation, discussion, comparisons, conclusions, perspectives
Acquired skills: To be able to propose a reaction mechanism accounting for a catalyzed redox reaction at the active site of a metallo-enzyme. To know how to analyze the experimental data, to interpret them.
Know how to interpret the results of studies based on isotopic effects and / or Hammett’s equation. Know how to propose an appropriate study method to elucidate a reaction mechanism.
Key words: redox reactions – metallo-enzymes – biomimetism- mechanism
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Maitrise des connaissances et concept de base en chimie de coordination.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: 50% Final written exam (2h) Oral presentation: 30%; continuous monitoring: 20%
![]()
![]()
Course Title: Advanced heterocyclic chemistry
Course supervisor:
- H. Dhimane
Description:
The module is an advanced organic synthesis course emphasizing the chemistry of heterocyclic compounds,
including:
– Synthesis and reactivity of micro-heterocycles (some 3 and 4 member rings)
– Synthesis of electron-deficient aromatic nitrogen heterocycles (pyridines, diazines and their benzo-fused analogues)
– Synthesis of electron-rich aromatic heterocycles (furans, pyrroles, thiophenes, azoles and their benzo-fused analogues)
Emphasis will be placed on the mechanistic aspects of the involved strategies, with a focus on recent methods involving
organometallic species. The importance of each family of heterocycles will be illustrated by examples from medicinal
chemistry. On completion of this course you are expected to:
– Become familiar with the main classes of heterocycles, especially aromatic ones
– Be able to explain at the mechanistic level the synthesis and the reactivity of the main heterocycles
– Devise synthetic pathways to (bioactive) compounds based on heterocyclic units
Key words: micro-heterocycles, heteroaromatics, synthesis and reactivity, selectivity, bioactive heterocycle.
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Maîtriser les données acquises au niveau du cycle licence, concernant la structure et les concepts de base de la réactivité des principales fonctions organiques.
Teaching methods and activities: Lectures (CM), Practical sessions (TD), autre: Projet personnel et/ou collectif
Assessment: Evaluation de l’exposé, Contrôle terminal
Course Title: Heteroelements in organic synthesis
Course supervisor:
- G. Prestat
Goals: The aim of this course is to study the reactivity and use of heteroelements in organic synthesis in a synthetic goal. The reactivity of Phosphorus, Sulfur, Silicon, Boron, Aluminium, Manganese, Chrome is presented and illustrated by single or double C-C and C-Het bond formation as well as oxidations and reductions of compounds organic.
Description:
Use of heteroelement for C-C and C-heteroatom bond formation.
Phosphorus: Arbusov, phosphonium salts synthesis, Wittig, Wittig horner, Mitsunobu, Corey-Fuchs …
Sulfur: thioketals, sulfonium and sulfoxonium ylides, beta anion to sulfur, sulfones synthesis and reactivity, sulfoxides…
Silicon: synthesis and reactivity of silyl-enol ethers, Peterson olefination, trialkylsilyl diazomethane reactivity…
Bore: boranes addition onto unsaturated C-C bond, reactivity
Use of heteroelement for organic molecules oxidation and reduction.
Oxidation: Swern, Moffat, Osmium …
Reduction: aluminum hydrides, boron hydride…
Acquired skills: Mastery of the concepts governing the reactivity of heteroelements conventionally used in organic synthesis.
Key words:
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Mastery of basic knowledge and concepts in inorganic and organic chemistry acquired L3 chemistry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final written exam 100%
Course Title: Organometallic chemistry
Course supervisor:
- H. Dhimane
Description:
* Formation, structure, stabilité et réactivité comparée des espèces organométalliques du « groupe principal »
* Réactivité des organométalliques stœchiométriques
– Formation et réactivité des organométalliques à base du Cuivre.
– Formation, structure et réactivité des énolates et analogues azotés (alkylation, aldolisation et réactions apparentées)
– Modèles de diastéréosélectivité simple et d’induction asymétrique par copule et pool chiral
(Zimmermann-Traxler, Evans, Enders, Cram-Chélaté, Cornforth, Felkin-Ahn …)
Acquired skills: Compréhension des concepts gouvernant la réactivité des organométalliques du «groupe principal». A l’issue de cet enseignement l’étudiant est à même de comprendre et d’exploiter la réactivité de ces espèces organométalliques dans la formation régio- et stéréo-sélective de liaisons C-C.
Key words: organométalliques stœchiométriques, structure, réactivité, sélectivités, formation de liaisons C-C.
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Bonne connaissance des concepts de base acquis en L3 (chimie) concernant la réactivité des principales fonctions en chimie organique.
Teaching methods and activities: Lectures (CM), Practical sessions (TD), autre: Projets personnels et collectifs
Assessment: Evaluation de l’exposé, Contrôle terminal
Course Title: Advanced NMR Spectroscopy
Course supervisor:
- N. Giraud
Goals: Presenting the concepts of the most recent and advanced methods that have been developed in Nuclear Magnetic Resonance in the main fields of application : analysis of solids and materials, nuclear spin relaxation, proteins, metabolomics. A description of modern techniques allowing for improving resolution, sensitivity and acquisition rate of NMR experiments will also be presented.
Description:
Solid-State NMR: Anisotropic interactions, magnetic susceptibility, magic angle spinning, HRMAS NMR, cross polarization, decoupling, recoupling, hardware, main 1D and 2D sequences.
Relaxation : Redfield theory, Solomon equations, spin-lattice and spin-spin relaxation mechanisms, cross-relaxation ,cross-correlationapplication to dynamics analysis in biomolecules.
Metabolomics: Story of the scientific development of metabolomics. Determination of biomarkers, personalized medecine, applications (vegetal, alimentation …) Data acquisition, data processing (statistical analysis, buckettinh, notion of variance, principal component analysis …), applications.
Protein NMR : triple-resonance experiments, application to structural and dynamics analysis. Study of protein-ligand interaction …
Improving resolution and sensitivity in NMR : pure shift NMR, J-resolved, hyperpolarisation, fast NMR
Acquired skills: The student will be able to choose the most informative and pertinent NMR methods to answer the questions raised by the molecular systems investigated in biomedical research.
Key words: Solid-State NMR, Relaxation, Metabolomics, Biomolecular NMR, Resolution and Sensitivity.
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: NMR lecture M1S1 UE8. 2D NMR, description of the main pulse sequences, bases of nuclear spin relaxation (T1, T2, nOe), chemical exchange.
Assessment: Contrôle terminal- 100% examen final
Course Title: Coordination Chemistry
Course supervisor:
- M. Etheve-Quelquejeu
Goals: The aim of this course is to address the specific problems for the synthesis of biomolecules (peptides, sugars and nucleic acids) based on general notions of organic chemistry.
Some chapters will be treated in the form of « Reverse Pedagogy ».
Description:
Sugars: Structural aspects (conformation,), monosaccharide chemistry (protective groups), formation of the O-glycosidic bonds (methods of activation, control of stereochemistry, mechanistic aspects).
Peptides: Formation of peptide bonds, strategy of synthesis, synthesis on solid support.
Nucleic acids: Protective groups, Phosphodiester bond formation, solid support synthesis.
Acquired skills: Elements of chemical reactivity encountered during the synthesis of biomolecules.
Key words: peptides, sugars and nucleic acids chemistry
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Bases in organic chemistry and molecular biochemistry
Teaching methods and activities: Lectures (CM), Practical sessions (TD), autres : Reverse Pedagogy
Assessment: Terminal exam 100%
![]()
![]()
Course Title: Introduction to Biotechnologies
Course supervisor:
- M-A. Sari
Goals: Acquérir les bases d’utilisation des biotechnologies autour des cours théoriques, illustrées par des travaux pratiques : clonage, expression et production d’une protéine recombinante et biotransformation d’un produit chimique par culture en fermenteur de microorganismes.
Description: Organismes genétiquement modifiés, cultures, clonage, utilisation de miccroorganismes en synthèse organique.
Acquired skills: Avoir au moins une fois réalisé une production d’enzyme recombinante de A à Z et/ou Realiser une biotransformation en fermenteur
Etre capable de discuter avec des biologistes en comprenant les problématiques
Key words: micro-organismes, clonage, biocatalyse, enzymologie
Total number of hours: 40 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Bases de biologie moléculaire : réplication, transcription, traduction, PCR (les cours enregistrés pourront être fournis au préalable aux étudiants n’ayant pas de notion de biologie moléculaire mais ces notions sont considérées comme acquises).
Bases d’enzymologie : cinétique michaelienne (impossible de suivre ce cours sans avoir des notions d’enzymologie)
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: contrôle continu : résultats et participation en travaux pratique sur une semaine en continu et évaluations théoriques écrites régulières pendant la semaine de TP.
ATTENTION LA PARTICIPATION EST OBLIGATOIRE ET PAS DE SESSION DE RATTRAPAGE POSSIBLE POUR LE TP
Course Title: Basis of bio-mollecular recognition
Course supervisor:
- V. Balland
Goals: To approach the structure/function relationships of simple biomolecules from the physicochemist’s point of view.
Description:
Part I : Introduction and Biomolecular Reminders
Composition, structure and stability of simple biomolecules: DNA, soluble and membrane proteins
Part II : DNA-ligand & DNA-protein interactions
Recognition mechanisms (intercalation, small-groove fixation, Pt complex), thermodynamic and kinetic aspects, Scatchard analysis, competitive processes, molecular basis of selectivity, structural consequences
Part III : Protein-ligand interactions
Ligand binding mechanisms (key-lock, induced-fit, conformational selection), thermodynamic and kinetic aspects, Scatchard analysis, cooperative concept, allosteric regulators, activation and enzymatic catalysis, ion channels.
Acquired skills:
- To transpose classical physicochemical concepts to biomolecules.
- To analyse biomolecular ligand binding processes by considering weak interactions and the associated kinetic and thermodynamic aspects.
- To reinforce the general biochemistry knowledge through case studies
Key words: Protein, DNA, supramolecular biochemistry
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Basics of kinetics and chemical thermodynamics.
Concepts of reactivity of molecular inorganic complexes.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: final exam (100%)
Course Title: Matériaux et Nanomatériaux fonctionnels
Course supervisor:
- B. Piro
Description:
Sont présentées les différentes familles de fonctionnalités applicables aux polymères et aux matériaux inorganiques (alliages métalliques, céramiques, semi-conducteurs) : fonctionnalités électriques, électroniques et applications (stockage et conversion d’énergie, transistors organiques, afficheurs, biocapteurs et capteurs médicaux implantables, matériaux magnétiques doux, optoélectronique). Les relations structure-propriété sont mises en avant.
Acquired skills: Connaissance de différentes fonctionnalités applicables aux matériaux tant inorganiques qu’organiques. Mise en œuvre de ces matériaux pour les applications industrielles modernes.
Key words: Polymères ; Matériaux inorganiques ; Fonctions ; Structures ; Propriétés
Total number of hours: 26 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed:
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: 60% CT: Contrôle Terminal / Final exam
40% CC: Contrôle Continu / Continuous assessment (TP + soutenance projet)
Course Title: Electrochimie et stockage de l’énergie
Course supervisor:
- M. Robert
Goals: L’objectif de cette UE est, à partir des notions fondamentales d’électrochimie acquise en M1S1, d’étudier et comprendre les grands processus de conversion et de stockage électrochimique de l’énergie solaire dans des matériaux et des molécules. Le cours inclura une partie sur la transduction de l’énergie puis seront abordées les questions de stockage électrochimique de l’énergie à l’aide de matériaux (supercondensateurs, accumulateurs) et de molécules (photosynthèse artificielle pour la production de carburants).
Description:
• Cellules photovoltaïques, Systèmes photosynthétiques naturels et artificiels (6h)
• Photosynthèse artificielle pour la production de carburants (8h)
• Condensateurs / supercondensateurs (4h)
• Accumulateurs électrochimiques : Piles et biopiles à combustible (4h)
• Séminaire (2h)
Acquired skills: Compréhension des mécanismes se produisant dans les systèmes photovoltaïques et les systèmes biomimétiques de transduction de l’énergie solaire ; Connaissance du principe de fonctionnement des différents systèmes électrochimiques de stockage de l’énergie et Capacité à évaluer les performances des générateurs électrochimiques ; Connaissance des principes de stockage de l’énergie par génération de carburants (photosynthèse artificielle).
Key words: Polymères ; Matériaux inorganiques ; Fonctions ; Structures ; Propriétés
Total number of hours: 24 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Electrochimie de M1S1
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Contrôle terminal (examen et présentation d’article)
![]()
Course Title: Elementary analysis and environment 1
Course supervisor:
- Fridolin Kwabia Tchana
Description: The purpose of this course is to acquire theoretical and practical basics of instrumentation in chemical analysis and to learn
how to quantitatively examine the state and operation of a complex multiphase medium like environment. This course is structured in 2
parts. The first part concerns the elementary analysis, the second part concerns the description of natural environments in order to
determine the functioning of the explored system, and the solutions implemented to study its chemical properties.
Key words: Elemental analysis, atom and plasma, spectroscopy, environment
Total number of hours: 30 Number of ECTS: 3 Semester 2
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed:Undergraduate degree in spectroscopies, analytical chemistry and environment
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: 60% CT: Contrôle Terminal / Final exam
40% CC: Contrôle Continu / Continuous assessment (TP + Vidéo)
![]()
![]()
Course Title: Foreign language & Professional skills
Goals: Anglais scientifique et préparation au TOEIC ou apprentissage du français
Description: enseignement obligatoire d’anglais de base et avancé pour l’étude de textesscientifiques et échanges internationaux et 10 à 20 h de préparation au TOEIC avec possibilité de se présenter au TOEIC par l’intermédiaire du Centre Technique de Langues
Acquired skills: savoir communiquer en Anglais dans un environnement scientifique. Être capable de lire et d’analyser un ouvrage scientifique en anglais.
Note : pour les étudiants anglophones, cette UE est remplacée par une UE d’apprentissage du français. Enseignement proposé par le Centre Technique de Langues.
Key words: apprentissage anglais ou français
Total number of hours: 20 Number of ECTS: 2 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: aucun
Assessment: contrôle continu (50 %) et examen final (50 %)
Course Title: Matériaux polymères
Course supervisor:
- B. PIRO
Description: Après une introduction aux polymères (définitions, régularités, degré de polymérisation, masse molaire) et aux techniques de caractérisation associées (exclusion stérique, viscosimétrie), seront présentées structure et propriétés chimiques et mécaniques, puis les méthodes de polymérisation : par étape, anionique, cationique, catalytiques, radicalaires et radicalaires contrôlées, et enfin les procédés de production (en masse, en suspension, en dispersion, en solution, en émulsion).
Acquired skills: Maîtrise des principales méthodes et procédés de synthèse et de caractérisation. Connaissance des principales propriétés, chimiques et mécaniques des matériaux polymères et copolymères. Apprentissage de la fonctionnalisation de surfaces par des polymères. Apprentissage du travail de documentation en équipe avec compte-rendu à l’oral, en langue anglaise.
Key words: Polymères ; Fonctions ; Structures ; Propriétés
Total number of hours: 26 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Connaissance des principaux groupes fonctionnels en chimie organique (niveau L2) ; Notions de cinétique chimique (niveau L3).
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: 60% CT: Contrôle Terminal / Final exam
40% CC: Contrôle Continu / Continuous assessment (TP + soutenance projet)
![]()
![]()
Course Title: Biological Chemistry
Course supervisor:
- O. Reinaud
Goals: Knowledge, at the molecular level, of the principles governing the reactivity of metal ions in biological media (transport, hydrolytic catalysis, redox catalysis) Reactive Mechanism at the Active Site
Description:
I. Presentation of the bio-inorganic domain
II. Regulation, transmission of information: alkaline and alkaline earth
III. Hydrolytic processes: hydrolysis of peptides, phosphodiesters, urea
IV. Electron transfer
V. Dioxygen transport and activation
Acquired skills: Knowledge of the main molecular tools available to a given biological system to achieve a given transformation. Know how to use the principles of coordination chemistry to propose a reaction mechanism in a biological environment. To master the formalism describing the exchanges of electrons during redox reactions.
Key words: Enzymes – Active site- mechanism
Total number of hours: 30 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Knowledge and basic concepts in chemistry (organic and coordination, acid-base catalysis, redox reactions) and biochemistry (amino acids, proteins, enzymes, active site, Michaelis–Menten)
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: 100% Final written exam (2h)
Course Title: Supramolecular chemistry
Course supervisor:
- B. Colasson
Goals: The teaching is fundamental and interdisciplinary: the aim is for the student to be able to acquire a general vision of the concepts non-covalent interactions in order to understand molecular processes (self-assembly, structures of complex chemical systems, molecular recognition) and to be able to create new assemblies (synthesis, recognition and detection).
Description:
– Historical background – first examples, emergence of the field: crown ether (Lehn, Pedersen, and Cram).
– Definitions: weak interactions, chemistry at the thermodynamic equilibrium.
– Overview of the spectroscopic and analytical methods
– Towards complex structures: a) different classes (helix, macrocycle, cage, foldamer, grid, etc…). b) topological chemistry (catenanes, rotaxanes, knots, etc…).
– Synthetic methods and template effect.
– Structure-function relationship.
– Molecular devices (molecular machines, sensors for anions or cations).
Acquired skills: In depth understanding of the roles of non-covalent interactions for studying molecular recognition in complex structures.
Key words: Basic knowledge and concepts in chemistry obtained in L3: thermodynamics, kinetics, coordination chemistry, atomistic (molecular orbital theory).
Total number of hours: 26 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Knowledge and basic concepts in chemistry (organic and coordination, acid-base catalysis, redox reactions) and biochemistry (amino acids, proteins, enzymes, active site, Michaelis–Menten)
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final exam 100 %
![]()
Course Title: Coordination Chemistry
Course supervisor:
- D. Over
Goals: This module is intended as a master level course covering transition metal and coordination chemistry. The aim is to provide an overview in bonding and spectroscopy, as well as an understanding of the reactivity of coordination compounds.
Description:
– Bonding (molecular orbital theory and crystal field), spectroscopy, magnetism
– Photochemistry, redox- and substitution reactions
Acquired skills: In the end of this course, a student should be able to fully understand the chemistry of the d-elements and be able to rationalize the trends of their chemical properties across the periodic table. With these tools in hand, a student should be able to interpret electronic and IR spectra, and to design synthetic strategies for coordination compounds as well as to evaluate their reactivity.
Key words: Coordination chemistry, transition metals, spectroscopy, magnetism, reactivity
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: General, inorganic and organic chemistry acquired in the Bachelor program.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final written exam. Depending on the number of the students, a presentation of scientific papers will be asked.
![]()
Course Title: Strategies in organic synthesis
Course supervisor:
- G. Prestat
Goals: Etre capable de définir des stratégies efficaces de synthèses de composés organiques de complexité moyenne.
Description:
– Principaux groupements protecteurs en synthèse organique.
– Eléments de rétrosynthèse :
disconnection, synthons, interconversion de groupements fonctionnels, addition de groupements fonctionnels.
Exemples de synthèse de composés d’intérêt biologique.
Acquired skills: A l’issue de cet enseignement les étudiants sont à même de définir une stratégie de synthèse de molécules organiques de complexité moyenne. Ils maitrisent les concepts de groupements protecteurs orthogonaux, et sont à même de repérer les disconnections les plus pertinentes. Ils maitrisent également le langage propre à la rétrosynthèse.
Key words: Groupements protecteurs, sélectivité, rétrosynthèse, stratégie
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Maitrise des connaissances et concept de base en chimie organique acquis en L3 Chimie.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Contrôle terminal 100%
![]()
![]()
Course Title: Orbitals, reactivity and mechanisms
Course supervisor:
- H. Dhimane
Description:
Théorie des orbitales moléculaires:
– Méthodes de Hückel et des OM de fragments pour la description des structures diatomiques, polyatomiques, conjuguées (acycliques et annulènes).
– Importance des OM pour l’analyse conformationnelle
– Effets stériques et stéréo-électroniques sur la stabilité et la réactivité
*Etude et description des mécanismes réactionnels : critères thermodynamique et cinétique, effets de substituants, étude de mécanismes (marquage et effets isotopiques, preuves stéréochimiques et cinétiques, effets du milieu, catalyse, effet de structure en phase gazeuse…), états de transitions, intermédiaires réactionnels.
* Etude des réactions péricycliques (électrocyclisations, sigmatropiques, cycloadditions, chélétropiques et transfert de groupes) par différentes méthodes (Dewar-Zimmermann, Woodward-Hoffmann, et Fukui).
Acquired skills:
– Maîtrise du concept des orbitales moléculaires pour une bonne appréhension de la structures et de la réactivité, avec une application dans le domaine des réactions péricycliques
– Aptitude à analyser et résoudre des problèmes techniques abstraits
– Aptitude à connecter et appliquer les concepts appris
– Aptitude à réfléchir et raisonner logiquement de manière critique et indépendante
Key words: orbitales moléculaires, réactivité, étude de mécanismes, réactions péricycliques.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Bonne connaissance des concepts de base acquis en licence concernant la structure et la réactivité chimiques.
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Projet personnel et/ou collectif
Assessment: Evaluation de l’exposé, Contrôle terminal
Course Title: From bonding to catalysis
Course supervisor:
- G. Prestat
Goals: This transition-metal catalysis course in homogeneous phase allows students to acquire mastery of basic concepts of this field. Particular emphasis is placed on the understanding of reaction mechanisms and metal-ligand interactions in order to determine the optimal reaction conditions. Heck reactions, palladium-catalyzed cross-couplings and olefin metathesis are studied in depth.
Description:
Coordination chemistry for organometallic catalysis
Elementary coordination chemistry: M-C and M-H bonds
Phosphine ligands, NHC carbene ligands
Pi-ligands: cyclopentadienyl, allyl, alkene, alkyne …
Elementary steps in organometallic chemistry: oxidative addition and reductive elimination, insertion and disinsertion, electrophilic and nucleophilic attack …
The general concept of catalysis and examples of the use of organometallic compounds in homogeneous catalysis: C-C couplings, Wacker process, olefin metathesis.
Organometallic catalysis
Mizoroki-Heck Coupling: Mechanism – regioselectivity – reactivity of halogenated and pseudo-halogenated partners – ligandless conditions – cyclizations – stereochemical aspects and asymmetric versions.
Cross-Couplings: Mechanistic aspects – Kumadu-Corriu – Negishi – Stille – Suzuki-Miyaura – Hiyama – Sonogashira
Olefin metathesis
Pd-catalyzed Allylic Alkylation
Acquired skills: Mastery of metal-ligand sigma and pi interactions and elementary steps in organometallic chemistry. Understanding of basic organometallic catalysis concepts in homogeneous phase. Mastery of palladium-catalyzed couplings and olefin metathesis.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Mastery of basic knowledge and concepts in inorganic and organic chemistry acquired L3 chemistry.
Teaching methods and activities: Lectures (CM), Practical sessions (TD)
Assessment: Final written exam (100 %)
Course Title: Solides inorganiques
Course supervisor:
- S. Ammar
Goals: L’objectif de cet UE est d’introduire les propriétés de transport dans le solide inorganique.
Description: Le contenu du cours proposé est le suivant :
– Les différents types de solides : comparaison en termes de cohésion et de conductivité électrique.
– Structure cristalline : métaux, modèle ionique, solides iono-covalents.
– Structure électronique des solides
Métaux : modèles des électrons libres et quasi-libres ;
Semi-conducteurs et composés ioniques : méthodes des orbitales moléculaires ;
Méthodes spectroscopiques d’études des niveaux d’énergie dans les solides.
– Défauts ponctuels dans les solides, non-stoechiométrie, dopage
– Transport de charges électriques :
Conductivité électronique dans les métaux et les oxydes ;
Conductivité électronique, électrolytes solides.
Méthodes de synthèse : méthodes céramiques, sol-gel, hydrothermales, CVD.
Cette ECUE sera composée de 18 h de cours, 8h de TD et 8 h de TP.
Total number of hours: 34 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed:
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: Examen final : 70 %, Travaux pratiques : 30 %
Course Title: Surfaces et interfaces
Course supervisor:
- J.-Y. PIQUEMAL
Goals: L’objectif de ce cours est d’abord de donner aux étudiants de solides bases en physico-chimie des surfaces et interfaces. Un accent particulier sera mis sur l’interface solide-gaz en présentant notamment une technique d’analyse propre à cette interface : la chromatographie en phase gazeuse.
Les connaissances acquises dans la première partie de ce cours seront mises à profit pour expliquer les mécanismes à la base de la formation de particules en phase liquide et leur stabilité tandis qu’un premier panorama des différentes techniques d’élaboration sera ensuite décliné. Ce cours constituera ainsi un préalable à une UE de M2, plus spécifiquement dédiée aux méthodes d’élaboration avancées s’étant développées récemment.
Description:
- Physico-chimie des surfaces et interfaces.
- Equation de Young-Laplace.
- Convention et isotherme de Gibbs.
- Equation de Kelvin.
- Mouillage des surfaces et capillarité.
- Adhésion et cohésion.
- Interface solide-gaz.
- Physi- et chimi-sorption. Les modèles de Langmuir et de Brunauer, Emmett et Teller.
- La porosité: définitions et méthodes d’évaluation.
- Application : la chromatographie en phase gazeuse.
- Interface solide-liquide : méthodes d’élaboration de matériaux à l’échelle nanométrique.
- Introduction à la synthèse de nanomatériaux.
- Synthèse d’oxydes, métaux et alliages en milieux aqueux ou non-aqueux.
- La voie sol-gel.
- Synthèses en milieu confiné.
- Synthèses hydrothermales.
Acquired skills: Comprendre les différents phénomènes se produisant aux interfaces. Avoir une bonne connaissance de base des différentes méthodes d’élaboration de matériaux par voie de synthèse en solution.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: connaissances de base en thermodynamique et en cinétique chimique
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: Contrôle terminal (60 %), TP (10 %), projet (30 %)
Une des spécificités de cet enseignement consistera en la mise en place de projets d’étude, menés en trinôme, portant sur l’une des méthodes d’élaboration vues dans le cadre de ce cours, et dont l’évaluation se fera via une présentation devant l’ensemble de la promotion.
Course Title: Surfaces et interfaces
Course supervisor:
- J.-Y. PIQUEMAL
Goals: L’objectif de ce cours est d’abord de donner aux étudiants de solides bases en physico-chimie des surfaces et interfaces. Un accent particulier sera mis sur l’interface solide-gaz en présentant notamment une technique d’analyse propre à cette interface : la chromatographie en phase gazeuse.
Les connaissances acquises dans la première partie de ce cours seront mises à profit pour expliquer les mécanismes à la base de la formation de particules en phase liquide et leur stabilité tandis qu’un premier panorama des différentes techniques d’élaboration sera ensuite décliné. Ce cours constituera ainsi un préalable à une UE de M2, plus spécifiquement dédiée aux méthodes d’élaboration avancées s’étant développées récemment.
Description:
- Physico-chimie des surfaces et interfaces.
- Equation de Young-Laplace.
- Convention et isotherme de Gibbs.
- Equation de Kelvin.
- Mouillage des surfaces et capillarité.
- Adhésion et cohésion.
- Interface solide-gaz.
- Physi- et chimi-sorption. Les modèles de Langmuir et de Brunauer, Emmett et Teller.
- La porosité: définitions et méthodes d’évaluation.
- Application : la chromatographie en phase gazeuse.
- Interface solide-liquide : méthodes d’élaboration de matériaux à l’échelle nanométrique.
- Introduction à la synthèse de nanomatériaux.
- Synthèse d’oxydes, métaux et alliages en milieux aqueux ou non-aqueux.
- La voie sol-gel.
- Synthèses en milieu confiné.
- Synthèses hydrothermales.
Acquired skills: Comprendre les différents phénomènes se produisant aux interfaces. Avoir une bonne connaissance de base des différentes méthodes d’élaboration de matériaux par voie de synthèse en solution.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: connaissances de base en thermodynamique et en cinétique chimique
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: Contrôle terminal (60 %), TP (10 %), projet (30 %)
Une des spécificités de cet enseignement consistera en la mise en place de projets d’étude, menés en trinôme, portant sur l’une des méthodes d’élaboration vues dans le cadre de ce cours, et dont l’évaluation se fera via une présentation devant l’ensemble de la promotion.
Course Title: Surfaces et interfaces
Course supervisor:
- J.-Y. PIQUEMAL
Goals: L’objectif de ce cours est d’abord de donner aux étudiants de solides bases en physico-chimie des surfaces et interfaces. Un accent particulier sera mis sur l’interface solide-gaz en présentant notamment une technique d’analyse propre à cette interface : la chromatographie en phase gazeuse.
Les connaissances acquises dans la première partie de ce cours seront mises à profit pour expliquer les mécanismes à la base de la formation de particules en phase liquide et leur stabilité tandis qu’un premier panorama des différentes techniques d’élaboration sera ensuite décliné. Ce cours constituera ainsi un préalable à une UE de M2, plus spécifiquement dédiée aux méthodes d’élaboration avancées s’étant développées récemment.
Description:
- Physico-chimie des surfaces et interfaces.
- Equation de Young-Laplace.
- Convention et isotherme de Gibbs.
- Equation de Kelvin.
- Mouillage des surfaces et capillarité.
- Adhésion et cohésion.
- Interface solide-gaz.
- Physi- et chimi-sorption. Les modèles de Langmuir et de Brunauer, Emmett et Teller.
- La porosité: définitions et méthodes d’évaluation.
- Application : la chromatographie en phase gazeuse.
- Interface solide-liquide : méthodes d’élaboration de matériaux à l’échelle nanométrique.
- Introduction à la synthèse de nanomatériaux.
- Synthèse d’oxydes, métaux et alliages en milieux aqueux ou non-aqueux.
- La voie sol-gel.
- Synthèses en milieu confiné.
- Synthèses hydrothermales.
Acquired skills: Comprendre les différents phénomènes se produisant aux interfaces. Avoir une bonne connaissance de base des différentes méthodes d’élaboration de matériaux par voie de synthèse en solution.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: connaissances de base en thermodynamique et en cinétique chimique
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: Contrôle terminal (60 %), TP (10 %), projet (30 %)
Une des spécificités de cet enseignement consistera en la mise en place de projets d’étude, menés en trinôme, portant sur l’une des méthodes d’élaboration vues dans le cadre de ce cours, et dont l’évaluation se fera via une présentation devant l’ensemble de la promotion.
Course Title: Surfaces et interfaces
Course supervisor:
- J.-Y. PIQUEMAL
Goals: L’objectif de ce cours est d’abord de donner aux étudiants de solides bases en physico-chimie des surfaces et interfaces. Un accent particulier sera mis sur l’interface solide-gaz en présentant notamment une technique d’analyse propre à cette interface : la chromatographie en phase gazeuse.
Les connaissances acquises dans la première partie de ce cours seront mises à profit pour expliquer les mécanismes à la base de la formation de particules en phase liquide et leur stabilité tandis qu’un premier panorama des différentes techniques d’élaboration sera ensuite décliné. Ce cours constituera ainsi un préalable à une UE de M2, plus spécifiquement dédiée aux méthodes d’élaboration avancées s’étant développées récemment.
Description:
- Physico-chimie des surfaces et interfaces.
- Equation de Young-Laplace.
- Convention et isotherme de Gibbs.
- Equation de Kelvin.
- Mouillage des surfaces et capillarité.
- Adhésion et cohésion.
- Interface solide-gaz.
- Physi- et chimi-sorption. Les modèles de Langmuir et de Brunauer, Emmett et Teller.
- La porosité: définitions et méthodes d’évaluation.
- Application : la chromatographie en phase gazeuse.
- Interface solide-liquide : méthodes d’élaboration de matériaux à l’échelle nanométrique.
- Introduction à la synthèse de nanomatériaux.
- Synthèse d’oxydes, métaux et alliages en milieux aqueux ou non-aqueux.
- La voie sol-gel.
- Synthèses en milieu confiné.
- Synthèses hydrothermales.
Acquired skills: Comprendre les différents phénomènes se produisant aux interfaces. Avoir une bonne connaissance de base des différentes méthodes d’élaboration de matériaux par voie de synthèse en solution.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: connaissances de base en thermodynamique et en cinétique chimique
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: Contrôle terminal (60 %), TP (10 %), projet (30 %)
Une des spécificités de cet enseignement consistera en la mise en place de projets d’étude, menés en trinôme, portant sur l’une des méthodes d’élaboration vues dans le cadre de ce cours, et dont l’évaluation se fera via une présentation devant l’ensemble de la promotion.
Course Title: Molecular modelling
Course supervisor:
- F. Maurel
Description: Cet enseignement s’adresse aux étudiants désireux de s’initier à la modélisation moléculaire, discipline de la chimie qui utilise les moyens informatiques pour représenter et déterminer des propriétés moléculaires. L’essentiel de l’enseignement utilise des logiciels de modélisation moléculaire incluant interface graphique pour construire les systèmes moléculaires et module de calculs de chimie orbitalaire pour déterminer des propriétés électroniques et modéliser la réactivité chimique (Logiciels Agui, Spartan ou Gaussian).
Les principaux concepts de la chimie théorique seront présentés : méthode de la fonction d’onde, exploration de la surface d’énergie potentielle, optimisation de géométrie, chemin de réaction et état de transition, modèle des orbitales frontières, calculs de spectres de vibration, introduction au calculs des états excités, modélisation de l’effet de solvant (méthode du continuum et de la supermolécule).
Une large place est faite à l’usage de ces logiciels pour illustrer des concepts de la chimie théorique sur des cas concrets et comparer les résultats obtenus à l’expérience.
Dans le détail cette UE abordera :
– L’étude de la structure électronique de molécules organiques à l’aide de la chimie orbitalaire : petites molécules diatomiques, triatomiques, molécules conjuguées.
– La réactivité moléculaire sur des réactions simples de la chimie organique (par exemple : substitution nucléophile, addition électrophile).
– La spectroscopie moléculaire : spectroscopie vibrationelle et UV-Vis.
Acquired skills: Principaux concepts de la chimie théorique : description de la liaison chimique, délocalisation électronique, modèle des orbitales frontières, surface d’énergie potentielle, optimisation d’une géométrie moléculaire, chemin réactionnel.
Utiliser un logiciel de modélisation moléculaire et mettre en pratique des calculs de chimie orbitalaire. Comprendre comment on calcule les diverses propriétés physico-chimiques du système à partir de sa fonction d’onde. En retirer les principales informations pour expliquer les propriétés moléculaires et la réactivité. Comparaison avec l’expérience.
Total number of hours: 30 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Il est préférable d’avoir suivi un enseignement de liaison chimique en Licence. Des connaissances en théorie des groupes sont un plus.
Teaching methods and activities: Lectures (CM), Practical sessions (TD), Lab sessions (TP)
Assessment: CT: Contrôle Terminal / Final exam
![]()
Course Title: Electrochemistry
Course supervisor:
- F. Mavre
Description:
This course has two specific objectives: (i) to introduce the key fundamental concepts required to understand and study the heterogeneous processes involving electron transfers and (ii) to introduce some of the main techniques used in the field of molecular electrochemistry (iii) to introduce some of the main contemporary applications of the electrochemistry in the fields of synthesis, energy materials and catalysis.
Part 1 (12 h) : After a short review about the main physicochemical properties of charged interfaces, the different processes associated to charge transfer reactions to an electrode, as well as the way to study them, will be detailed. The course will provide students with solid theoretical skills in the following topics:
- Thermodynamics of an electrochemical cell
- Solution transport processes
- Introduction to electrochemical kinetics
- Electrochemical methods (chronoamperometry, rotating disk electrode, cyclic voltammetry).
Part 2 (6 h): The purpose of this second part will be to give an overview of some important applications of electrochemistry, related to synthesis (redox economy, biomass valorization, …) on one hand and to processes aiming at solving energy challenges on the other hand (electrochemical storage of renewable energies, solar fuels). It will be completed by one or two seminars given by researchers from the industry or academic institutions.
Practicals (2 x 3 h = 6 h) The practical work sessions will focus on the use of cyclic voltammetry for the analysis of electrochemical and electrocatalytic processes involving the electrode material or a molecular species.
Total number of hours: 24 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Assessment: Final Exam: 70 % Practical Works: 30 %
![]()
Course Title: NMR
Course supervisor:
- N. Giraud
Goals: Providing students with the concepts and theoretical tools to understand pulsed NMR (1D and 2D). Teaching them how to analyze, exploit, and discuss the analytical content of NMR spectra to determine the structure of organic molecules (either natural or synthetic), and notably biomolecules.
Description: After a brief review of the fundamentals of this spectroscopy, this lecture will introduce the main features of NMR pulse sequences. A simplified theoretical formalism based on Bloch equations will be developed to describe and understand the the evolution of macroscopic nuclear magnetization under different regimes (precession, relaxation, spin echo …-).
Heteronuclear NMR will also be addressed : the sensitivity and the quality of the data that are available for a selection of heteronuclei in the field of organic chemistry will be discussed. Multi-dimensional NMR will also be introduced, and the fundamental concepts of multi-dimensional data acquisition and processing will be introduced for 2D pulse sequences. The main homo- and hetero-nuclear correlation experiments will be presented (COSY, TOCSY, NOESY, HSQC).
Finally, nuclear spin relaxation will be introduced, as well as chemical exchange. Tutorials will allow for completing theoretical concepts with exercises focused on applications, and analyses of spectra recorded on real samples (structure determination from experimental spectra).
Practicals will allow students for applying the acquired knowledge to the acquisition of data targeting dynamic and/or structural analysis.
Acquired skills:
– assimilate / explain / discuss the theoretical concepts of NMR: acquisition of a 1D and 2D NMR experiment, relaxation mechanism, nOe, dyanmics …
– deciphering and implementing a pulse sequence
– analyzing 2D maps recorded on synthetic or natural molecules to determine their structure.
Total number of hours: 32 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Fundamentals of NMR: magnetic properties of the nucleus, Zeeman effect, chemical shift, scalar spin-spin coupling, basic principles of the analysis of a 1D 1H spectrum.
Assessment: Contrôle terminal- 100% examen final
![]()
Course Title: Basis of surface functionalization
Course supervisor:
- G. Mattana
Goals: This Teaching Unit provides the students with the fundamentals of surface functionalization both from a physical and a
chemical point of view.
Description: The TU is divided into two parts: a first part describing the basic concepts of surfaces and their physico-chemical properties (3 hrs) as well as the most important physical functionalization methods (thermal evaporation, CVD-ALD, spin-coating, applications to photolithography – 6 hrs). The second part (9 hrs) deals with chemical functionalization methods. Self-assembly
processes and chemisorption for the formation of molecular monolayers will be described as well as the elaboration of thin layers via post-functionalization of self-assembled monolayers or direct electrografting of small molecules up to conducting polymers. Chemical and physical properties and applications of selected examples will be discussed.
Prerequisites/skills needed: Bases of chemical reactivity in organic and inorganic chemistry; bases of spectroscopy and electrochemistry (level bachelor).
Total number of hours: 18 Number of ECTS: 3 Semester 1
Mandatory course ☒ Optional course ☐
Prerequisites/skills needed: Bases of chemical reactivity in organic and inorganic chemistry; bases of spectroscopy and
electrochemistry (level bachelor).
Assessment: Contrôle terminal – 100% examen final
